Article 3 (final)
I am a member of Community Voice Australia- communityvoiceaustralia.org. We put in a submission to FSANZ raising serious concerns regarding their support of the first Lab food in to the Australia and New Zealand market. The full submission will be put on the website in coming weeks. You can also email me for a copy. Here are some excerpts:
Our submission primarily covers the following areas:
1. Transparency and accountability
2. Health concerns
3. Public knowledge and Behavioural Modification techniques
Transparency and Accountability
It is troubling that the public is unable to see a vast swathe of information related to Vow Food Lab Quail procedures, inputs and processes. And it begs the question as to how the public can have genuine input in to the FSANZ Hazard and Risk assessment?
Vow requests the information contained within the following appendices be considered confidential commercial information (CCI):
· ● Appendix 3 Mycoplasma Reports [CCI]
· ● Appendix 4 Sterility Report [CCI]
· ● Appendix 5 Retrovirus Report [CCI]
· ● Appendix 6 Species Specific Bacteria Report [CCI]
· ● Appendix 7 Species Specific Virus Report [CCI]
· ● Appendix 8 Microbiological Reports [CCI]
· ● Appendix 9 Heavy Metal Reports [CCI]
· ● Appendix 10 Antibiotic Reports [CCI]
Appendices 3-10 provide confidential details on Vow’s cell supplier and their cell line. Non-confidential descriptions of these appendices are provided in section B.4.
· ● Appendix 11 Manufacturing Process [CCI]
· ● Appendix 12 Veterinary Certificate [CCI]
· ● Appendix 13 HACCP Plan [CCI]
· ● Appendix 14 GCCP Plan [CCI]
Novel Food – Vow Cultured Quail
Appendices 11-14 provide confidential details pertaining to Vow’s manufacturing process. Non-confidential descriptions of these appendices are provided in section B.5.
Appendix 15 Species Confirmation [CCI]
Appendix 15 provides confidential details on Vow’s cell supplier and their cell line. A
non-confidential description of this appendix is provided in section C.6.1.2.
· ● Appendix 18 Allergen Reports [CCI]
· ● Appendix 19 Laboratory Accreditations [CCI]
· ● Appendix 20 Gross Composition and Nutrient Testing Methods [CCI]
· ● Appendix 21 Proximate Reports [CCI]
· ● Appendix 23 Amino Acid Statistics [CCI]
Appendices 18-23 provide confidential details about the components of Vow’s cultured quail product. Non-confidential descriptions of these appendices are provided in sections C.6.1.4 and C.6.2.
· ● Appendix 24 Reagent CoAs [CCI]
· ● Appendix 25 Reagent and Processing Aid Risk Assessment [CCI] [CCI]
· ● Appendix 26 Residue Measurements [CCI]
· ● Appendix 27 Growth Factor CoAs [CCI]
· ● Appendix 28 Growth Factor Reports [CCI]
· ● Appendix 29 Genetic Stability [CCI]
Appendices 24-29 provide confidential details on the components used in the media. Non-confidential descriptions of these appendices are provided in section C.6.3.
· ● Supplemental Appendix 1 Microbiological Results [CCI]
· ● Supplemental Appendix 2 Proximate Reports [CCI]
· ● Supplemental Appendix 3 Amino Acid Stats [CCI]
· ● Supplemental Appendix 4 Residue Measurements [CCI]
· ● Supplemental Appendix 5 Reagent and Processing Aid Risk Assessment [CCI]
· ● Supplemental Appendix 6 Growth Factor #2b CoA and Background Reports [CCI]
· ● Supplemental Appendix 7 Growth Factor Results [CCI]
· ● Supplemental Appendix 8 Allergen Reports [CCI]
· ● Supplemental Appendix 9 Bioinformatic Reports [CCI]
Supplemental Appendices 1-9 provide confidential details on the testing and analyses conducted on Vow cultured quail manufactured with Growth Factor #2b. Non-confidential descriptions of these appendices are provided in the Supplement, “FSANZ Supplemental Update”.
This is blatantly non transparent. Transparency and accountability must be a mandatory core of the regulatory process. In a regulatory environment based on a novel synthetic product being placed in the food market there must be forensic audit trails which can place direct financial and criminal liability, not just on corporations at multiple points in the supply chain, but also on entire boards of directors, and top-ranking senior managers.
For any food supply industry which markets products with short histories of usage and safety testing, there must be a licensing regime, where no product can go to market unless it has sign-off by boards of directors and senior management and liens on assets. The burden of risk must not be shouldered by the consumers, but by the vendors. If anything goes wrong, the burden of proof lies not with the consumer to prove harmfulness, but on the vendor to prove safety.
We are unable to find the names of the people sitting on the FSANZ Novel food committee, we feel it is only appropriate that whatever final decision is made by the committee that decision makers are identified, in the interests of transparency and accountability. To accept a Novel Food into the market, without any prior health trials also demands a level of accountability to any detrimental flow on effects.
Safety and Health Concerns
The FSANZ summary states that the reason for accepting Vow Food’s submission is because it “relates to a matter that warrants the variation of a food regulatory measure”.
The reasoning for this statement is not clarified by FSANZ (please correct us if we missed it).
We are assuming that you are referring to the two primary justifications used by Biotech companies, chemical companies, venture capital firms, vegan lobby groups, and global bodies such as the United Nations and their partners, to garner support for Lab Created Cell products which can be sold as food. They are:
United Nations Sustainable Development Goal 2- Zero Hunger
United Nations Sustainable Development Goal 13- Climate Action.
Biotech company Vow Food’s application to FSANZ to amend the Australia New Zealand Food Standards Code to allow for the sale of “Cultured Quail” in Australia and NZ states as the justification for their submission:
The human population is projected to grow to 9.7 billion by 2050 (UN, 2022). Rising incomes along with rising population will increase global demand for protein by over 40%. As leading global exporters of protein, Australia and New Zealand will need to develop new technologies to help meet the domestic and international increases in demand.
Though SDG13- Climate Action is not addressed in the application. Vow Founder George Peppou is quoted often stating Lab created Cell Products that can be sold as food (hereafter called Lab Meat), are imperative to save the Climate/ Environment and lower emissions. https://greekherald.com.au/news/australia/vow-foods-cofounder-george-peppou-says-their-lab-grown-meat-is-more-environmentally-friendly/
The science on whether Lab Meat is better for the climate than conventional agriculture has not been thoroughly studied, particularly in the scale up projections needed to feed the world.
Interest in animal cell-based meat (ACBM) or cultured meat as a viable environmentally conscious replacement for livestock production has been increasing, however a life cycle assessment for the current production methods of ACBM has not been conducted. Currently, ACBM products are being produced at a small scale and at an economic loss, however ACBM companies are intending to industrialize and scale-up production. This study assesses the potential environmental impact of near term ACBM production.https://www.biorxiv.org/content/10.1101/2023.04.21.537778v1.full
We will not discuss further the environmental concerns regarding mass scale energy intensive Lab Meat. However, we will discuss the Feeding the world aspect. If Lab Meat can scale up, and it is intended to replace traditional and organic agriculture, FSANZ has a responsibility to ensure it is safe in large quantities.
When reading both Vow Food’s Submission and FSANZ response there is no real discussion on the impact on human health, no studies on the cumulative effect of synthetic end to end processes and their effect on the human body. There is an assumption that because biological Quail is safe to eat, that Lab Quail is safe to eat.
Vow Culture Quail Submission states:
E. Information on the nutritional and health impact of the novel food
E.1 Information to demonstrate that the use of the novel food or novel food ingredient will not cause a nutritional imbalance in the diet
A comprehensive literature search was conducted and did not identify any published literature to suggest that consumption of cultured quail would be associated with nutritional imbalance in the diet.
E.2 Information to demonstrate that the addition of the novel food ingredient will not create a significant negative public health impact
Not applicable; the purpose for adding a novel food ingredient does not relate to a potential beneficial physiological or health-related outcome. A comprehensive literature search was conducted and did not identify any published literature to suggest that consumption of cultured quail would be associated with negative public health impact…
Lab Quail has never been served as food, and we are not aware of any trials on the health effects of people eating Lab Quail. Given, most people are unaware of what Lab meat is or how it is produced, how can there be literature regarding the effects of eating it or how the public will respond to Lab Quail?
The same bait and switch technique of replacing biological quail with Lab quail is repeated through Vow Food’s submission and FSANZ’s response. Biological quail is used as the baseline for the safety of the Lab quail. Lab quail is compared to biological quail in terms of vitamins, protein, fat, carbohydrates, amino acids etc. There is no discussion or studies on the difference between naturally occurring vitamins, protein, fat, carbohydrates vs synthetically added ones. There is no mention of the cumulative effect of the numerous layers (how many? We are not told) of synthetic additives to create the Lab quail meat.
References on the variation between naturally sourced vitamins vs synthetic vitamins
Barringer, T.A., et al. (2000). Effects of a multivitamin/mineral supplement on micronutrient status, antioxidant capacity, and immune function in elderly individuals. Nutrition Journal, 16(9), 715-725. This study found that individuals who consumed a multivitamin/mineral supplement derived from natural sources had improved nutrient absorption and antioxidant capacity compared to those who consumed a synthetic supplement.
Davy, B.M., et al. (2004). Food form and portion size affect postprandial appetite sensations and hormonal responses in healthy, nonobese, older adults. Obesity Research, 12(3), 562-568. This study demonstrated that consuming whole foods with naturally occurring nutrients led to better appetite control and hormonal responses compared to consuming the equivalent nutrients in a synthetic form.
Alshiek, J., et al. (2018). Natural and synthetic vitamin E: Roadblocks in translational research. Annu Rev Nutr, 38(1), 181-206. This review highlights the challenges in translating findings from studies on synthetic vitamin E to natural vitamin E due to differences in absorption, metabolism, and bioavailability.
Haskell, C.F., et al. (2017). The effects of lutein and zeaxanthin supplementation on cognitive function in healthy older adults: A systematic review of randomized controlled trials. Journal of Functional Foods, 38, 247-258. This review found that supplementation with natural sources of lutein and zeaxanthin, such as leafy greens and eggs, led to greater cognitive benefits compared to synthetic forms.
Storcksdieck Genannt Bonsmann, S., et al. (2008). Effect of the form in which vitamin C is consumed on postprandial serum ascorbate concentration in healthy human subjects. British Journal of Nutrition, 99(04), 862-865. This study showed that naturally occurring vitamin C in orange juice led to higher postprandial serum ascorbate concentration compared to synthetic vitamin C supplements.
FSANZ Hazard and Risk Statement outlines:
While there is no history of consumption of cultured quail cells as food there is a long history of safe consumption of quail meat and eggs. Evaluation of the cell media and other inputs used during the production process demonstrated there are no safety concerns from exposure to these substances from consumption of the harvested cells. The available information indicates the harvested cells are unlikely to pose a food allergenicity concern for the general population.
And there is the oft repeated caveat that because people won’t be eating much of this Lab Quail that FSANZ is not concerned about the nutritional risks.
At what point (how much Lab Food approved in to the Australian and NZ food market?) will FSANZ remove this caveat (niche market) from their approvals for Lab Meats, given the stated trajectory of Lab Meat competing with or replacing meat?
We also note that in FSANZ Hazard and risk assessment it states:
Vow confirmed that all materials used in the production process meet the requirements for food grade or pharmaceutical grade ingredients with a purity and quality suitable for their intended use in food. The processing conditions are designed for food production following Hazard Analysis and Critical Control Point (HACCP) principles supported by good practices. The production process in this application consists of preparation and maintenance of cell banks (master and working), cell expansion (seed train) and cell harvesting. Currently no independent microbiological data or specifications exist to assess the hazards of this particular food against. Additionally, it is still a new area for which no such criteria have been established internationally.
Counter Article “Lab-grown meat is supposed to be inevitable. The science tells a different story” Outlines the concerns regarding contamination:
If even a single speck of bacteria can spoil batches and halt production, clean rooms may turn out to be a basic, necessary precondition. It may not matter if governments end up allowing cultured meat facilities to produce at food-grade specs, critics say—cells are so intensely vulnerable that they’ll likely need protection to survive. https://thecounter.org/lab-grown-cultivated-meat-cost-at-scale/
Given there is no microbiological data to assist the safety risk and hazards of Lab Quail, we propose that a trial study must be commenced by Vow Food, for an appropriate length of time to establish that there are no detrimental effects on human health. We are concerned regarding the establishment of legal requirements regarding complete disposal of contaminated cell lines and deep cleaning of all areas of factories, and the high likelihood of contamination of cell cultures during production (it only takes a unicellular bacteria or a non-cellular virus to completely distort original samples)
Health concerns would need to look at concerns such as "Leaky gut", which is an inflammatory condition of the intestinal epithelium. This epithelial layer is a vital barrier in the process of nutrient absorption and prevention of macro proteins eg food substrates that have not been properly processed, bacteria & other microorganisms or other toxins from passing into circulation. These proteins, if not fully broken down, can trigger systemic inflammation as the body's immune system recognises them as 'foreign' and acts to destroy and eliminate them.
Leaky gut has increased significantly over the last few decades due to a combination of higher consumption of processed foods, medications & stress, all of which directly affect the diversity and balance of the gut microbiome that is vital in maintaining endothelial integrity.
Among other issues, a great concern regarding the introduction of inadequately-tested lab-generated 'foods' is the impact that these substances will have on the gut microbiome, the gut epithelial barrier and how this will allow direct access into the body of foreign modified proteins.
There appears to have been no research conducted regarding Lab Meat’s effects in people already experiencing higher levels of systemic inflammation and chronic diseases.
References:
Fasano, A. (2012). Zonulin, regulation of tight junctions, and autoimmune diseases. Annals of the New York Academy of Sciences, 1258(1), 25-33.
Camilleri, M. (2019). Leaky gut: mechanisms, measurement and clinical implications in humans. Gut, 68(8), 1516-1526
Bischoff, S. C., et al. (2014). Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterology, 14(189).
Growth factors
Vow Food submission states that two recombinant growth factors are added to media during production to support the growth of the quail cells. One recombinant growth factor (referred to as Growth Factor #1) is sourced from barley seed and contain the purified recombinant growth factor along with selected barley seed proteins. This is genetically engineered pig growth factor and produced in barley seed.
For the second, Vow may interchangeably use one of two similar recombinant growth factors: One of these (referred to as Growth Factor #2a) has a similar manufacturing profile to the first growth factor. It is sourced from barley and the media additive contains the purified recombinant growth factor along with select barley seed proteins.
The second (referred to as Growth Factor #2b) is sourced from an Escherichia coli (E. coli) BL21 (DE3) strain and contains only the purified recombinant growth factor.
Recombinant DNA is the general name for a piece of DNA that has been created by combining two or more fragments from different sources.
Again, it’s concerning that the public is unable to see what is in the growth serum due to Confidential Commercial Information clauses. Considering the Lab Quail is made using Recombinant DNA how can Vow Food state their Lab Quail is non-GMO?
FSANZ Risk statement states Whilst there are numerous inputs contained in the basal media used for production, FSANZ considers these inputs low risk and after having regard to the definition of "used as a processing aid" in 1.1.2—13, does not consider them processing aids. That is, they do not perform a technological purpose in the course of processing. This consideration aligns with our assessments of food enzymes and other specialty foods where the basal media inputs are not deemed processing aids. Whilst not intending to insert these inputs into the Code in this instance, FSANZ has assessed each input to ensure their safety (section 3.1 of SD1). The majority of inputs in the basal media are permitted in the Code as amino acids, vitamins or minerals, processing aids or food additives.
FSANZ has not addressed the risks and hazards inherent in the recombinant growth factors. Why?
Spontaneous Immortalisation
The public is not able to assess the process whereby Vow Food keeps the Quail cells in a Spontaneous Immortalisation state, due to Confidential Commercial Information clauses. Due to concerns regarding cell immortalisation technically being a pre- cancerous or cancerous state, what risk analysis did FSANZ undertake or what information did FSANZ rely on in their consideration?
Health concerns excess Vitamins
FSANZ risk and hazard report states that there are very high levels of synthetic vitamins in the Lab Quail. This is assessed as non-concerning due to the Lab Quail being used in small quantities. For future Lab Meat assessments, at what point in the market saturation will you look at the cumulative effects of high synthetic vitamin/ mineral levels in Lab Meat in your assessment process?
Public knowledge and Behavioural Modification techniques
FSANZ’s Consumer Insights Tracker (surveyed roughly 2000 Australian and NZ residents) results found only 6 percent of people surveyed knew enough about Lab Meat to be able to explain it to someone else. Not surprising, given the number of steps and processes that Lab Meat must be put through.
Additionally, the report states that Consumers’ generally have low levels of awareness of cell-cultured meat, as most consumers (74%) have either never heard of cell-cultured meat or have heard of it but know very little or nothing about it. This is not surprising, given that cell-cultured meat is currently not available for sale in Australia and New Zealand.
The vast majority of the Australian and NZ public is unaware of Lab Meat and what it entails. This is not assisted by Lab Meat being represented (as included in FSANZ documentation) like this:
FSANZ Consumer literature review states:
Although the term ‘lab-grown’ enabled consumers to correctly identify the product, it has lower levels of perceived safety than other terms.
And
However, consumer perceptions of the healthfulness/nutritional value of cell-cultured meats appear to be highly malleable depending on the information received (neutral vs. biased descriptions) and product categories compared (chicken/beef vs. chicken nuggets/beef burgers).
And
Although the term ‘lab-grown’ enables consumers to correctly identify the product as not being farmed/fished/wild-caught, it has lower levels of perceived safety
And
Qualitative findings suggest that levels of trust in scientists, experts and/or cell- cultured meat companies may impact perceptions of the healthfulness and/or nutritional equivalence of cell-cultured meat. That is, those participants who had confidence in those involved in the production process had confidence that they would make it equivalent to conventional meat on these measures, and vice versa.
It reads as though FSANZ, is aware that Lab grown is the language that assists people to correctly identify Lab food products yet are concerned that people do not perceive this as safe. Why is FSANZ concerning itself with people’s perception of Lab Food, rather than people being able to correctly be able to identify the product? Why is FSANZ concerned regarding the malleability of people’s perceptions of Lab Meat depending on language? How is this within the scope of FSANZ’s responsibilities?
Vow Food submission refers to people who do not want to try Lab Meat as having Food Neophobia (the fear or dislike of new things). We vehemently disagree with the pathologising of people’s wariness of synthetic food, particularly when Vow Food’s processes are hidden under confidential commercial information clauses.
FSANZ Consumer Literature Review further states:
Qualitative findings suggest that levels of trust in scientists, experts and/or cell- cultured meat companies may impact perceptions of the healthfulness and/or nutritional equivalence of cell-cultured meat. That is, those participants who had confidence in those involved in the production process had confidence that they would make it equivalent to conventional meat on these measures, and vice versa.
We suggest that people may work out for themselves if they do or don’t trust Lab Food if there are:
1. Long term trials before being released in to the Australian and NZ food market.
2. Forensic Audit Trails at every step in the process.
3. Ability for people to see all the chemicals, processes, technology, additives, scaffolding equipment, medium that is used.
4. That Lab Meat processes are not dumbed down and instead explained appropriately.
5. That vested interests stop using language, and framing questions using behavioural modification techniques.
We will complete our submission with excerpts, from the UN FAO Food safety aspects of cell-based foodReport, outlining the steps needed to create Lab Meat:
1. Cell sourcing
2. Cell isolation
3. Cell storage
4. Cell proliferation
5. Cell differentiation
6. Process design for large scale production
7. Harvesting
8. Food processing
https://www.who.int/publications/i/item/9789240070943
The extensive, complicated, and highly synthetic procedures outlined by the United Nations bodies FAO and WHO are in stark relief to the information the public is given by either Vow Food or the FSANZ for the submission process. To make informed choices regarding Lab Meat the public must have transparent information available to them, including the specific details on the processes and additions at each stage of the synthetic Lab Meat process. The public must also be assured that the appropriate people are held to account if any health hazards and risks occur due to the release of the Lab Meat in to the Australian and NZ food market.
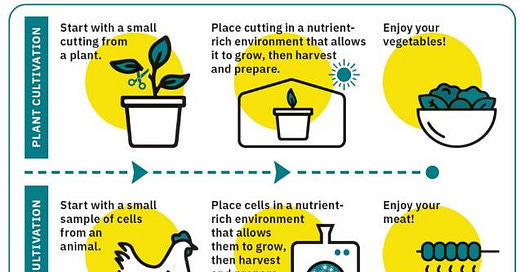


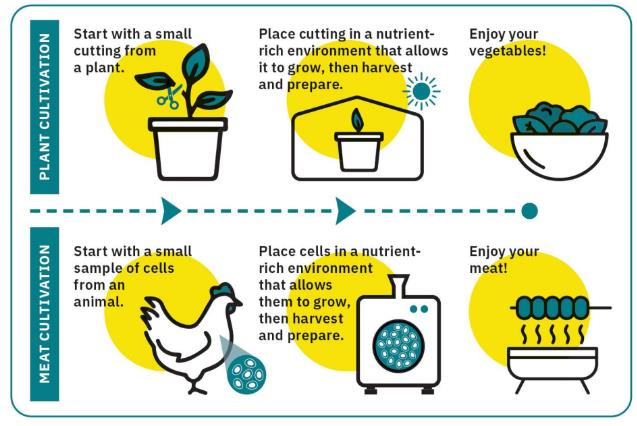
... and that is 4th Industrial Revolution food. Can we go back to the dark ages please?
All owners, board members & investors of Vow should be put on a daily diet of Vow lab grown quail for 2 years before the product is allowed for sale to the public. If they are alive & have had no health issues, then it may be allowed into the marketplace.
Out of curiosity what were some examples of the non-confidential information that was included in their application?